Nanostructured anodic TiO2 and WO3 modified/doped with transition metal oxides as photoanodes for electrocatalytic and sensing applications
Project coordinator: Grzegorz Sulka
Duration: 2017-2020
The main objective of this project is to develop and optimize the fabrication of doped/modified nanostructured anodic metal oxides (TiO2 and WO3) with enhanced photoelectrochemical and sensing properties. We expect that doping/modifying of semiconductor nanostructures with another semiconductor material, based on transition metal (e.g., Co, Fe, Cu) oxides, makes them promising candidates for next generation photoelectrochemical applications. In general, three different approaches will be undertaken to achieve this goal: (i) in-situ doping of anodic oxides during anodization of Ti and W foils in electrolytes containing transition metal ions, (ii) post-anodizing electrodeposition of transition metal/metal oxides on the surface of formed nanostructured anodic TiO2 and WO3, and (iii) wet impregnation of anodic oxides with solutions containing transition metal ions.
Details of our research can be found in the following publications:
K. Syrek, M. Sołtys-Mróz, K. Pawlik, M. Gurgul, G.D. Sulka, Photoelectrochemical properties of annealed anodic TiO2 layers covered with CuOx, Molecules 21 (2022) 4789.
In this work, we present a systematic study on the influence of Cu2+ ion concentration in the impregnation solution on the morphology, structure, optical, semiconducting, and photoelectrochemical properties of anodic CuOx-TiO2 materials. Studied materials were prepared by immersion in solutions with different concentrations of (CH3COO)2Cu and subjected to air-annealing at 400 °C, 500 °C, or 600 °C for 2 h. The complex characterization of all studied samples was performed using scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), reflectance measurements, Mott–Schottky analyses, and photocurrent measurements. It was found that band gap engineering based on coupling CuO with TiO2 (Eg~3.3 eV) is an effective strategy to increase the absorption in visible light due to band gap narrowing (CuOx-TiO2 materials had Eg~2.4 eV). Although the photoactivity of CuO-TiO2 materials decreased in the UV range due to the deposition of CuO on the TiO2 surface, in the Vis range increased up to 600 nm at the same time.
M. Zych, K. Syrek, M. Pisarek, G.D. Sulka, Anodic WO3 layers sensitized with hematite by electrodeposition and annealing method operating under the visible light spectrum, J. Power Sources 541 (2022) 231656.
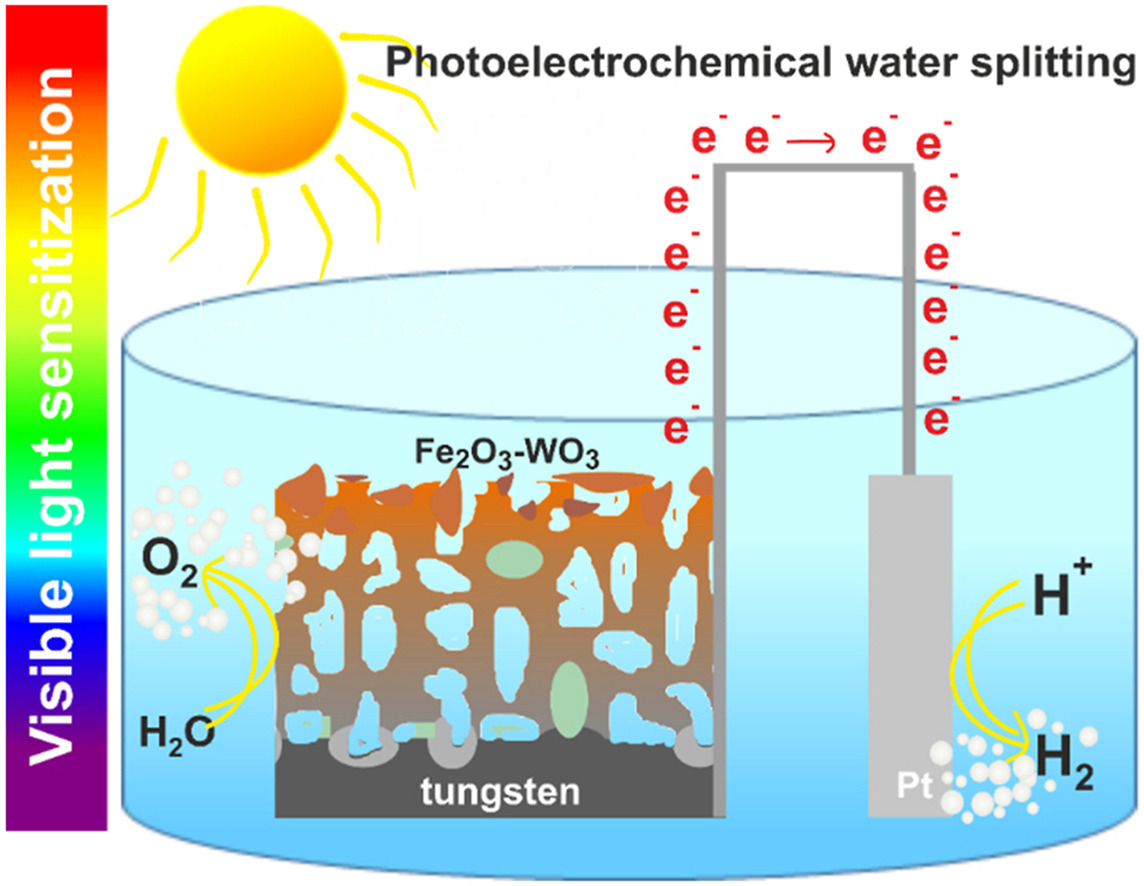
Tungsten oxide has been considered one of the most promising photoanode materials for photoelectrochemical (PEC) water splitting. WO3 possesses a relatively narrow band gap (2.5–2.8 eV) and superior charge transport properties. In this paper, for the first time, anodic WO3 layers were sensitized with Fe2O3 by electrodeposition and re-annealing method. The influence of FeCl3 concentration in the electrolyte used for the electrodeposition process on the morphology, semiconducting and photoelectrochemical properties was investigated. Since the band gap of hematite is narrower when compared to tungsten oxide, its modification/sensitization with hematite is a promising way to transfer WO3 photoactivity to the visible light region. To sum up, a successfully performed synthesis of Fe2O3-WO3 and Fe2O3–C,N-WO3 heterojunctions resulted in a shift of the material photoresponse towards the visible light region even to the wavelength of 550 nm. The best results were achieved for the Fe2O3–C,N-WO3 sample, that exhibits IPCE values about 1.6 times higher at 450 nm and 12 times higher at 480 nm in comparison to pristine anodic WO3.
K. Syrek, M. Zych, G.D. Sulka, Tuning the visible light activity of tungsten oxide layers by changing the anodization conditions, J. Ind. Eng. Chem. 112 (2022) 316-322.
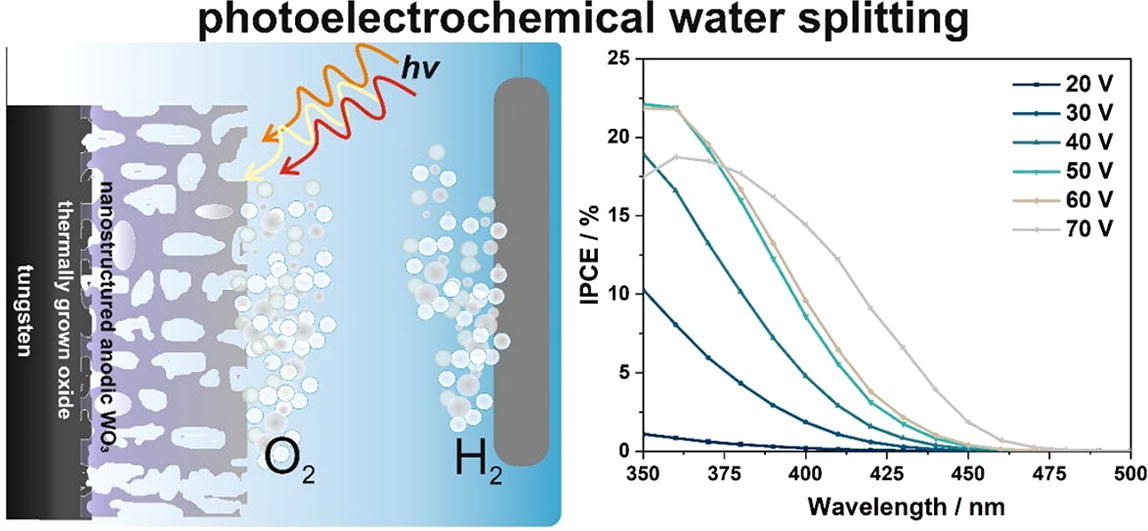
Photoelectrochemical water splitting under solar radiation is one of the most studied methods of solar-to-fuel energy conversion. The search for effective photoelectrode materials operating under solar radiation is still ongoing, and in this paper, it is shown how to control the optical and photoelectrochemical properties of anodic tungsten oxide layers by changing the anodizing potential. Anodic tungsten oxide layers were obtained in an aqueous electrolyte with 1 M ammonium sulfate and 75 mM ammonium fluoride at a constant voltage of 20–70 V and annealed at 500 °C in air. Comprehensive characterization of materials was performed using SEM, XRD, UV–Vis DRS, Mott-Schottky analyses, and photoelectrochemical water splitting tests. It was found that with increasing the anodizing potential the band gap narrows from 3.09 eV to 2.75 eV, donor density increases, and the IPCE spectrum shifts towards the visible light range.
M. Sołtys-Mróz, K. Syrek, Ł. Pięta, K. Malek, G.D. Sulka, Photoelectrochemical performance of nanotubular Fe2O3–TiO2 electrodes under solar radiation, Nanomaterials 12 (2022) 1546.

Fe2O3–TiO2 materials were obtained by the cathodic electrochemical deposition of Fe on anodic TiO2 at different deposition times (5–180 s), followed by annealing at 450 °C. The effect of the hematite content on the photoelectrochemical (PEC) activity of the received materials was studied. The synthesized electrodes were characterized by field emission scanning electron microscopy (FE-SEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), Raman spectroscopy, diffuse reflectance spectroscopy (DRS), Mott–Schottky analysis, and PEC measurements. It was shown that the amount of deposited iron (ca. 0.5 at.%–30 at.%) and, consequently, hematite after a final annealing increased with the extension of deposition time and directly affected the semiconducting properties of the hybrid material. It was observed that the flat band potential shifted towards more positive values, facilitating photoelectrochemical water oxidation. In addition, the optical band gap decreased from 3.18 eV to 2.77 eV, which resulted in enhanced PEC visible-light response. Moreover, the Fe2O3–TiO2 electrodes were sensitive to the addition of glucose, which indicates that such materials may be considered as potential PEC sensors for the detection of glucose.
M. Zych, K. Syrek, M. Pisarek, G.D. Sulka, Synthesis and characterization of anodic WO3 layers in situ doped with C, N during anodization, Electrochim. Acta 411 (2022) 140061.
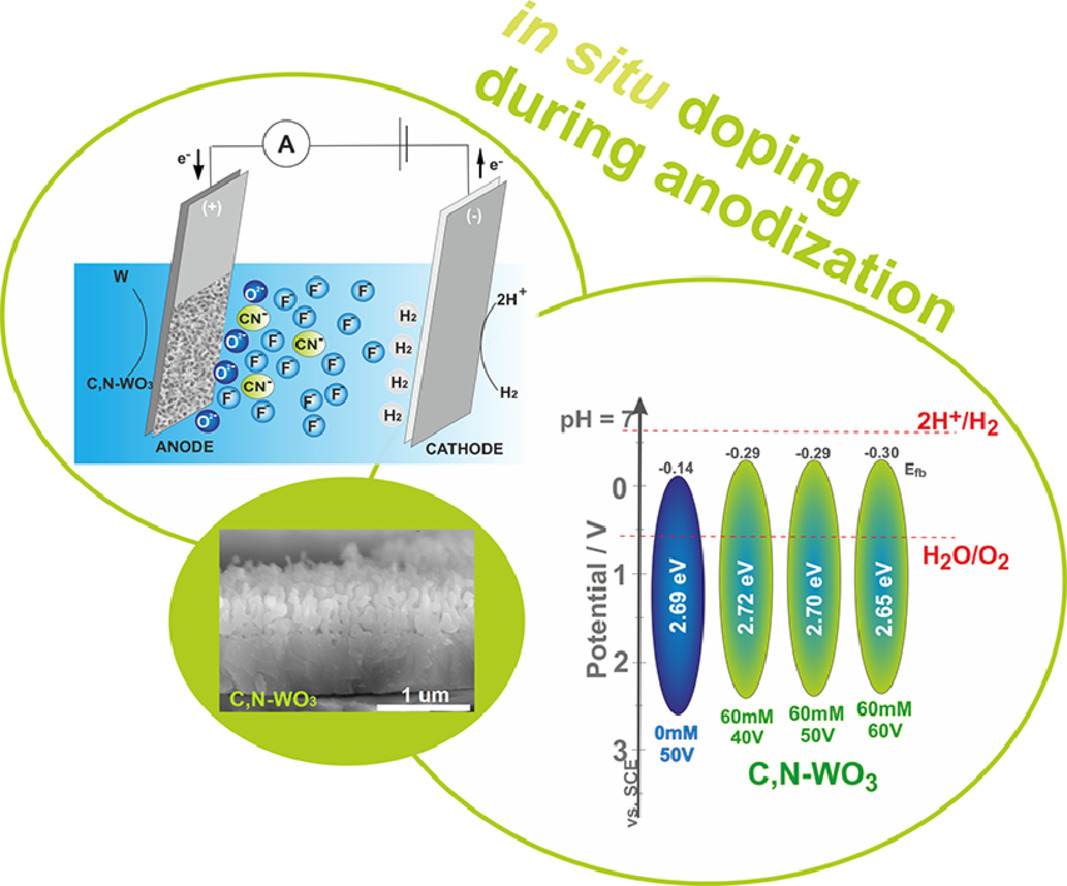
In this work, for the first time, anodic C,N-WO3 layers were obtained by in situ doping during anodic oxidation of metallic tungsten. The effects of applied potential (40, 50, and 60 V), and hexacyanoferrate(III) ion concentration (0, 15 and 60, 120, and 180 mM) in the electrolyte on the resulting oxide morphology, phase composition, and photoelectrochemical performance were investigated. To obtain a photoactive oxide phase, as-received samples were annealed at 500 oC in air for 2 h. The synthesized materials were subjected to complex physicochemical characterization using SEM, EDS, XRD, and XPS. In addition, the semiconducting properties of prepared samples were characterized using the Mott-Schottky analysis. The anodic C,N-WO3 layers were used as photoanodes for water splitting experiments. The best photoelectrochemical performance was observed for the samples obtained in 1 M (NH4)2SO4 and 0.075 M NH4F with 120 mM K3[Fe(CN)6] at 50 V for 4 h.
M. Sołtys-Mróz, K. Syrek, E. Wiercigroch, K. Małek, K. Rokosz, S. Raaen, G.D.Sulka, Enhanced visible light photoelectrochemical water splitting using nanotubular FeOx-TiO2 annealed at different temperatures, J. Power Sources 507 (2021) 230274.
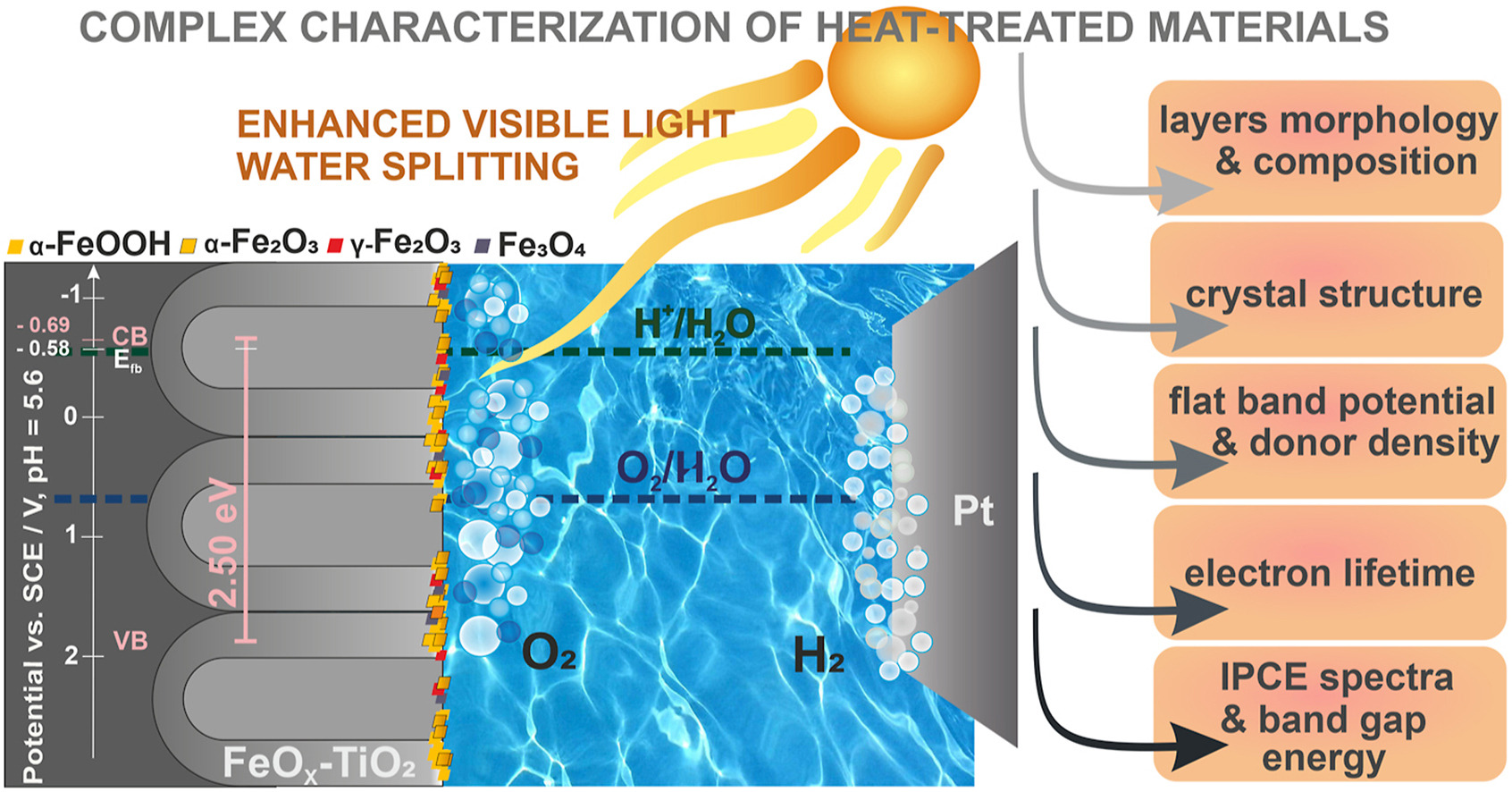
Nanostructured semiconductor photoelectrodes that can operate under renewable solar energy are of great interest for both fundamental studies and practical applications. In this paper, we studied the semiconducting and photoelectrochemical properties of nanotubular FeOx-TiO2 annealed at different temperatures. Nanostructured anodic titanium oxide layers were modified by impregnation with a solution containing 100 mM FeCl3 followed by their annealing at 400°C, 500°C, and 600°C. The synthesized materials were characterized by using a field emission scanning electron microscopy (FE-SEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy (RS), diffuse reflectance spectroscopy (DRS), electrochemical impedance spectroscopy (EIS), Mott-Schottky analysis, and photoelectrochemical measurements. It was found that depending on the annealing temperature, the studied materials showed a different photoelectrochemical response. The lowest value of band gap was observed for the sample annealed at 500°C, which is related to the presence of a mixture of anatase and rutile phases together with iron oxides.
M. Zych, K. Syrek, E. Wiercigroch, K. Malek, M. Kozieł, G.D. Sulka, Visible-light sensitization of anodic tungsten oxide layers with CuWO4, Electrochim. Acta 368 (2021) 137591.
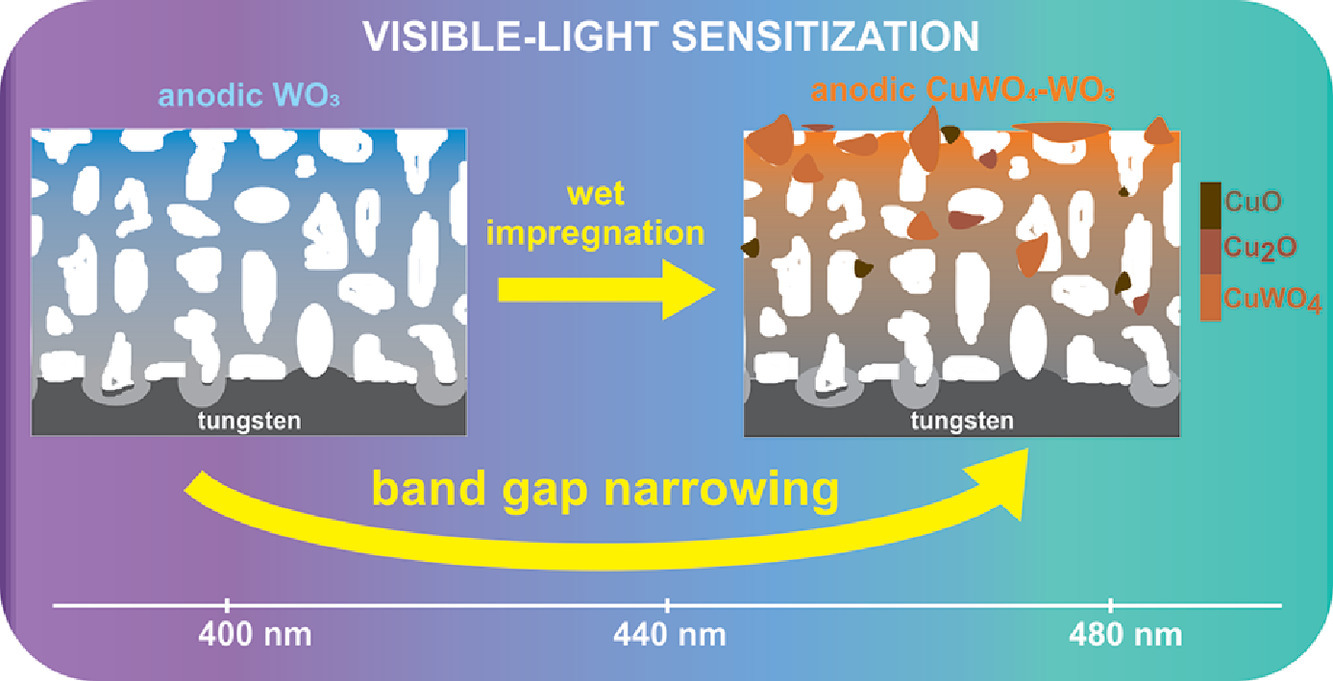
Anodic tungsten oxide is an attractive material for photoelectrochemical water splitting under solar irradiation, however due to several limitations, it absorbs only UV light and, therefore, one of the challenges is to transfer its photoresponse to the visible light region. In this paper, for the first time, it is proposed sensitization of anodic WO3 layers with copper tungstate by a wet impregnation method. The CuWO4-WO3 materials are obtained by immersion of anodic layers in a copper acetate solution and annealing at 500 oC in air atmosphere. As-received materials are characterized by scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction analysis (XRD), and Raman spectroscopy, Mott-Schottky measurements and photoelectrochemical (PEC) tests. Proposed visible-light sensitization with CuWO4 results in an enhanced photoelectrochemical response to visible light. Moreover, narrowing of the band gap (from 2.88 to 2.47 eV) with a prolonged impregnation time is also observed.
M. Zych, K. Syrek, L. Zaraska, G.D. Sulka, Improving photoelectrochemical properties of anodic WO3 layers by optimizing electrosynthesis conditions, Molecules 25 (2020) 2916.

Although anodic tungsten trioxide has attracted increasing attention in recent years, there is still a lack of data on influence of different electrolytes used for anodization on semiconducting and photoelectrochemical properties of obtained tungsten oxide layers. In this work, the attempt to correlate how different anodizing conditions (especially electrolyte composition) affect the surface morphology, thickness, and photoelectrochemical properties of anodized oxide layers was presented. Samples anodized in various electrolytes were subjected to comprehensive characterization by scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction analysis (XRD), photoelectrochemical (PEC) and Mott-Schottky measurements. As expected, the surface morphology of WO3 depends strongly on anodizing conditions. Annealing as-synthesized tungsten oxide layers at 500 oC for 2 h leads to obtaining a monoclinic WO3 phase in all cases. From Mott-Schottky analysis, it has been confirmed that all as prepared anodic oxide samples are n-type semiconductors. Band gap energy values estimated from incident photon-to-current efficiency (IPCE) measurements neither differ significantly for as-synthesized WO3 layers nor depend on anodizing conditions such as electrolyte composition, time and applied potential. Although the estimated band gaps are similar, photoelectrochemical properties are different because of many different reasons including the layer morphology (homogeneity, porosity, pore size, active surface area), oxide layer thickness, and composition of electrolyte used for anodization. Based on the conducted research, it was found that WO3 with a well-defined porous morphology and the best PEC properties are formed by anodization carried out in 1 M (NH4)2SO4 and 0.075 M NH4F at 50 V for 4 h.
M. Sołtys-Mróz, K. Syrek, J. Pierzchała, E. Wiercigroch, K. Małek, G.D. Sulka, Band gap engineering of nanotubular Fe2O3-TiO2 photoanodes by wet impregnation, Appl. Surf. Sci. 517 (2020) 146195.
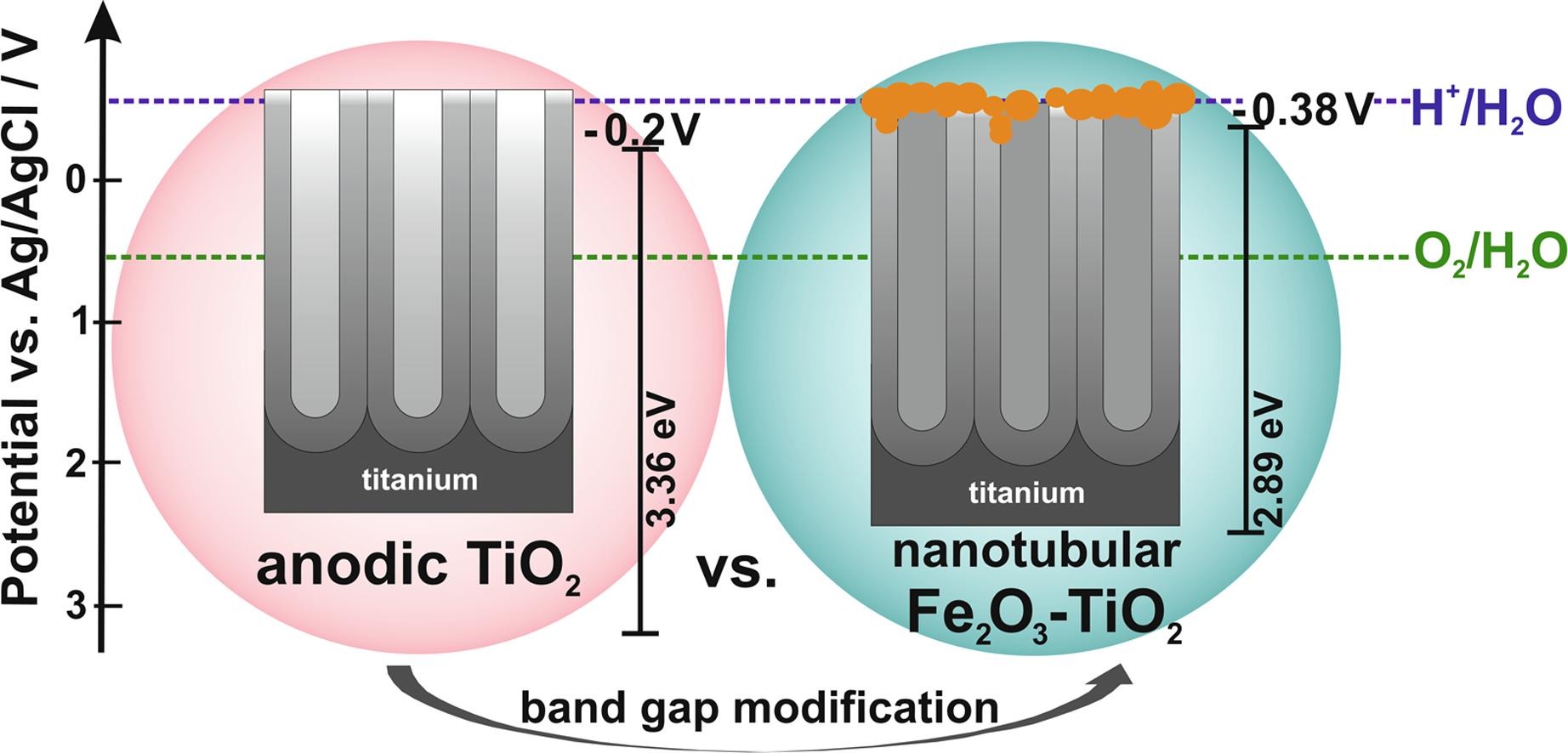
Due to many limitations of titanium oxide, its direct use in photoelectrochemical water splitting under solar radiation is ineffective. Band gap engineering based on introduction of the FeOx phase into TiO2 by wet impregnation has been proposed. In particular, nanoporous anodic titanium oxide layers were soaked in solutions with different concentrations of iron ion (5 – 100 mM) followed by their air annealing at 400 °C. As-prepared nanotubular FeOx-TiO2 materials were characterized by using field emission scanning electron microscopy (FE-SEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), reflectance measurements, Mott-Schottky analysis, and photoelectrochemical tests. It was found that increasing the iron content in anodic materials strongly affects their resulting semiconducting and photoelectrochemical properties. The maximum of generated photocurrent shifts towards visible light as the band gap energy decreases (from 3.36 to 2.89 eV) with the respect to increasing the iron content in the anodic materials.
K. Syrek, M. Skolarczyk, M. Zych, M. Sołtys-Mróz, G.D. Sulka, A photoelectrochemical sensor based on anodic TiO2 for glucose determination, Sensors 19 (2019) 4981.
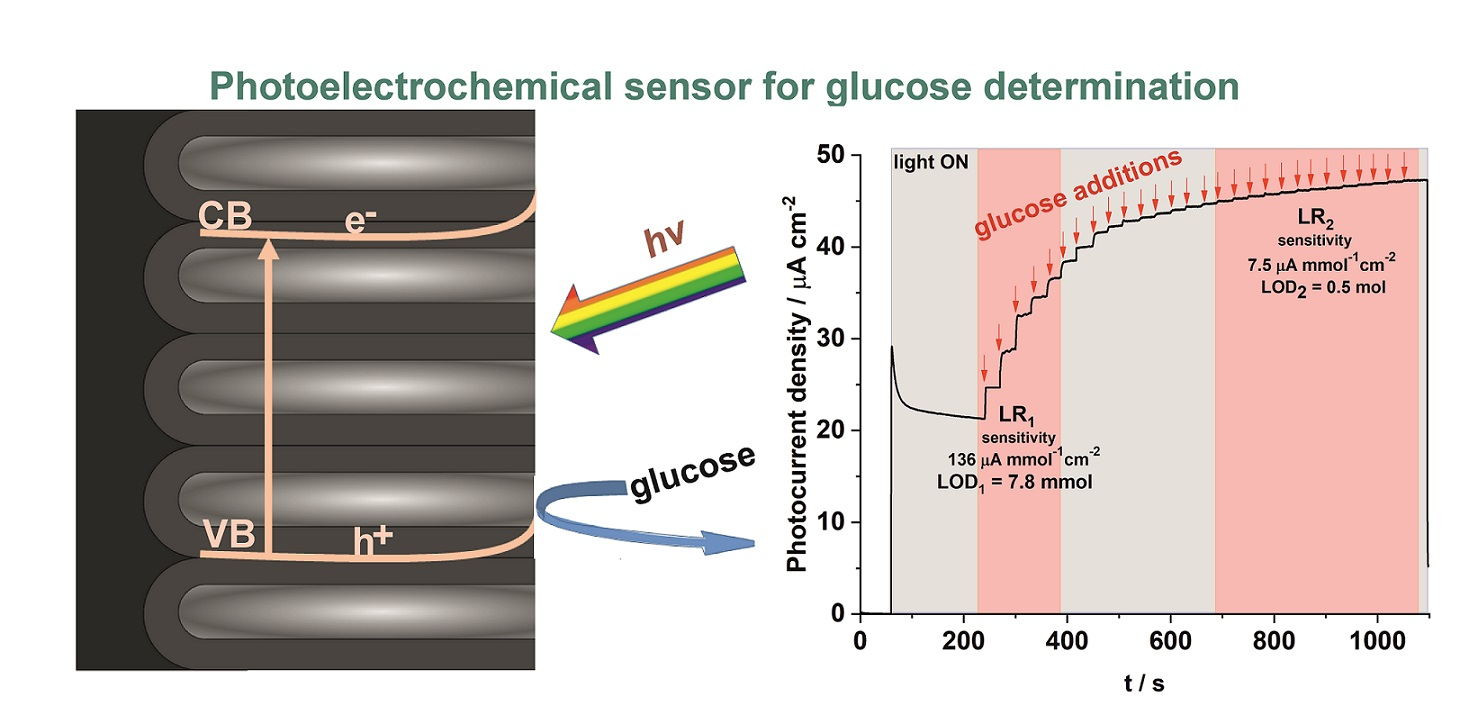
A simple PEC sensor based on non-modified nanostructured anodic TiO2 was fabricated, and used for a rapid and sensitive detection of glucose. The anodic TiO2 layers were synthesized in an ethylene glycol-based solution containing NH4F (0.38 wt.%) and H2O (1.79 wt.%) via a three-step procedure carried out at the constant voltage of 40 V at 20 °C. At the applied potentials of 0.2, 0.5 and 1 V vs. SCE, the developed sensor exhibited photoelectochemical response toward the oxidation of glucose, and two linear ranges in calibration plots were observed. The highest sensitivity of 0.237 µA µmol−1 cm−2 was estimated for the applied bias of 1 V. The lowest limit of detection (LOD) was obtained for the potential of 0.5 V vs. SCE (7.8 mM) with the fastest response ~ 3 s. Moreover, the proposed PEC sensor exhibited relatively high sensibility, good reproducibility, and due to its self-cleaning properties a good long-term stability. Interfering tests showed selective response of the sensor in the presence of urea and uric acid. Real-life sample analyses were performed using an intravenous glucose solution which confirmed the possibility of determining the concentration of analyte in such types of samples.
K. Syrek, L. Zaraska, M. Zych, G.D. Sulka, The effect of anodization conditions on the morphology of porous tungsten oxide layers formed in aqueous solution, J. Electroanal. Chem. 829 (2018) 106-115.
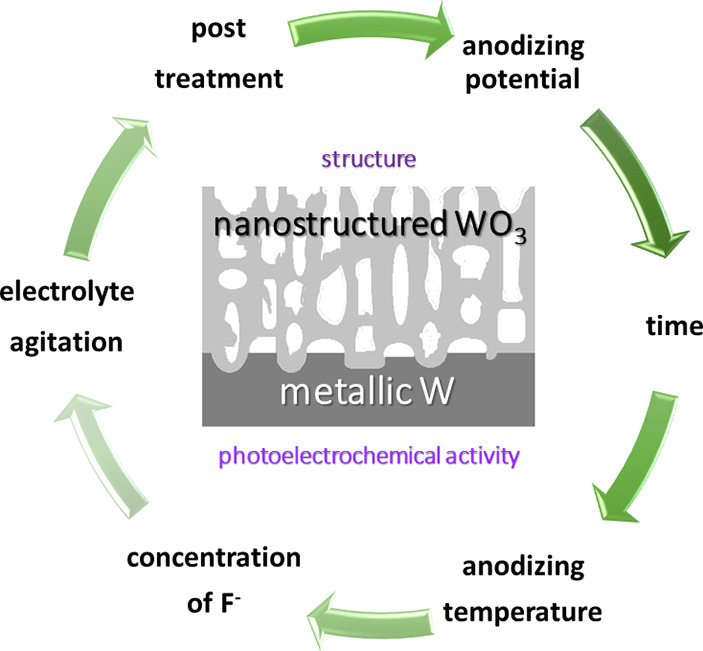
Nanostructured WO3 layers were obtained by one-step anodic oxidation of tungsten in an aqueous solution containing fluoride ions. A detailed investigation of the growth of anodic films on metallic substrate was focused on the study of the influence of anodizing conditions, such as: anodizing potential, duration of the process, electrolyte composition, applied temperature, and electrolyte agitation on morphological features of as-received materials. Such kind of comprehensive studies has been performed for the first time. It was found that the optimal conditions allowing synthesis of nanostructures with well-defined pores and the thickest possible oxide layer are: anodizing potential of 50 V, anodizing time of 4 h, electrolyte containing 1 M ammonium sulfate and 75 mM ammonium fluoride, temperature of 20 °C, and agitation of electrolyte of 250 rpm. A post-treatment procedure was developed to effectively remove the precipitates formed on the surface of anodic layers during anodization. The obtained materials were also examined as photoanodes in photoelectrochemical (PEC) water splitting experiments. It was confirmed, that PEC performance of nanostructured WO3 photoanodes can be significantly improved (higher photocurrents by 24 %) after ultrasonic treatment in a 20 wt.% HF solution for 10 s and ethanol for 5 s.