Nanostrukturalne elektrody półprzewodnikowe typu rdzeń-powłoka o ściśle określonej geometrii - projektowanie, otrzymywanie i charakterystyka fotoelektrochemiczna
Kierownik projektu: Leszek Zaraska
Okres realizacji: 2019-2025
Rosnące zapotrzebowanie na energię, jak również stopniowe wyczerpywanie się zasobów paliw kopalnych sprawia, że opracowanie nowych technologii pozwalających na uzyskanie czystych, łatwo dostępnych i odnawialnych źródeł energii stało się w ostatnim czasie jednym z podstawowych wyzwań współczesnej nauki. W tym kontekście, szczególne zainteresowanie wzbudza gazowy wodór, będący niezwykle obiecującym i przyjaznym środowisku paliwem o wysokiej gęstości energii.
Dotychczas zaproponowano wiele metod otrzymywania gazowego wodoru. Jednakże, zdecydowana większość z nich wymaga stosowania paliw kopalnych lub dużych nakładów finansowych. Dlatego w ostatnim czasie uwaga świata naukowego skupia się na możliwości otrzymywania wodoru z wody z wykorzystaniem energii elektrycznej. W 1972 r. Fujishima i Honda po raz pierwszy wykazali, że rozkład wody można również przeprowadzić w procesie fotoelektrolizy. Od tego momentu zainteresowanie procesem fotoelektrochemicznego rozkładu wody stale rośnie. Jednakże, zastosowanie ogniw fotoelektrochemicznych do otrzymywania wodoru na dużą skalę nadal wymaga rozwiązania wielu istotnych problemów ograniczających wydajność tego rodzaju urządzeń.
Projekt zakłada przeprowadzenie szeregu badań podstawowych prowadzących w efekcie do opracowania metody otrzymywania nowego typu nanostrukturalnych fotoelektrod (fotoanod i fotokatod) w formie uporządkowanych struktur nanodrutów SnO2/TiO2, ZnO/TiO2 oraz Cu2O/SnO2 typu rdzeń-powłoka o różnych morfologiach. Najbardziej innowacyjnym aspektem projektu będzie zastosowanie nowej metody elektrochemicznego osadzania wykorzystującej nanoporowate szablony Al2O3 w celu wytworzenia nanodrutów o różnym kształcie (prostych, o modulowanych średnicach, w kształcie litery Y, oraz periodycznie rozgałęzionych) na powierzchni przewodzących podłoży szklanych. W ramach projektu dokonana zostanie również kompleksowa charakterystyka nanostrukturalnych fotoelektrod na każdym etapie ich otrzymywania, a morfologia i skład otrzymanych materiałów zostaną skorelowane z ich właściwościami fotoelektrochemicznymi.
Spodziewamy się, że tego rodzaju struktury nanodrutów typu rdzeń-powłoka o unikalnej morfologii będą doskonałymi materiałami na fotoelektrody wykorzystywane w procesie fotoelektrochemicznego rozkładu wody.
Szczegóły przeprowadzonych przez nas badań można znaleźć w poniższych publikacjach:
L. Zaraska, M. Szuwarzyński, A. Świerkula, A. Brzózka, The effect of Al polishing conditions on the growth and morphology of porous anodic alumina films, ACS Omega 8 (2023) 34564-34574.

The conditions applied during the electrochemical polishing of aluminum were found to be important parameters for the successive formation of nanoporous alumina films. First, a high-purity Al foil was electrochemically polished in an aqueous solution containing C2H5OH and HClO4 at various sets of conditions, such as applied potential (5−35 V), temperature (0−20 °C), and process duration (10−180 s). Extensive studies of the topography of Al after polishing by scanning electron microscopy and atomic force microscopy allow verification of the correlations between conditions applied during the substrate pretreatment and dimensions of the nanopatterns generated on the metal surface. Next, Al polished samples at two different sets of conditions were used as starting materials for anodization. Unpolished Al samples were also anodized for reference. It was confirmed that electropolishing conditions do not significantly affect the oxide growth rate during anodization and the efficiency of anodic film formation. On the contrary, it was proved that the dimensions of the surface texture formed during Al polishing significantly affect the morphology and pore order within the anodic film. Therefore, it can be stated that it is possible to tune to some extent the arrangement of nanochannels within anodic aluminum oxide films by simply changing conditions during the electropolishing procedure.
K. Syrek, O. Tynkevych, M. Wojtas, M. Kozieł, Ł. Pięta, L. Zaraska, Room-temperature electrochemical deposition of nanostructured ZnO films on FTO substrate and their photoelectrochemical activity, J. Ind. Eng. Chem. 126 (2023) 171-180.
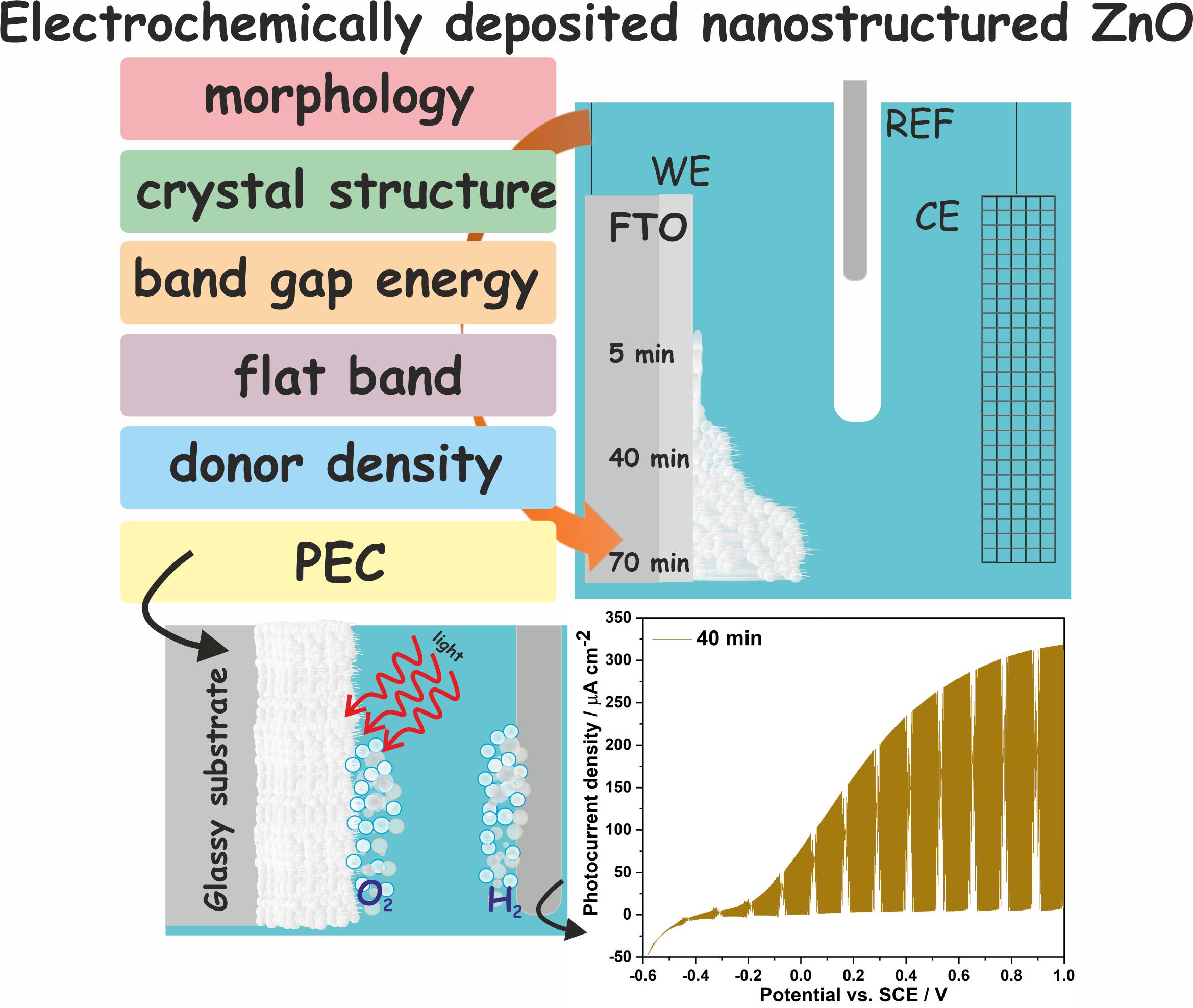
The possibility of fabrication of nanostructured ZnO layers on conductive glass substrates via simple room-temperature electrodeposition was studied in detail. The process was carried out in zinc nitrate electrolyte at different potentials for various durations. The morphology, composition, and semiconducting properties of the deposits were examined by various techniques. As-synthesized materials were found to be amorphous and co-deposition of the metallic Zn at more negative potentials was confirmed. Nevertheless, thermal treatment in air results in the successful conversion of the as-deposited precipitate to the wurtzite ZnO. The potential of −1.5 V vs. SCE was identified as optimal to achieve both full coverage of the glass substrate, as well as uniform morphology of the deposits. In general, the photoelectrochemical performance of such kind of ZnO photoanodes was found to be significantly dependent on the electrodeposition duration since this parameter strongly affected the thickness, surface morphology, as well as semiconducting properties of the obtained material. In consequence, the photoanode deposited at −1.5 V vs. SCE for 40 min exhibited the most promising photoelectrochemical activity under studied conditions.
K. Syrek, M. Jażdżewska, M. Kozieł, L. Zaraska, Photoelectrochemical activity of Cu2O electrochemically deposited at different temperatures, J. Ind. Eng. Chem. 115 (2022) 561-569.
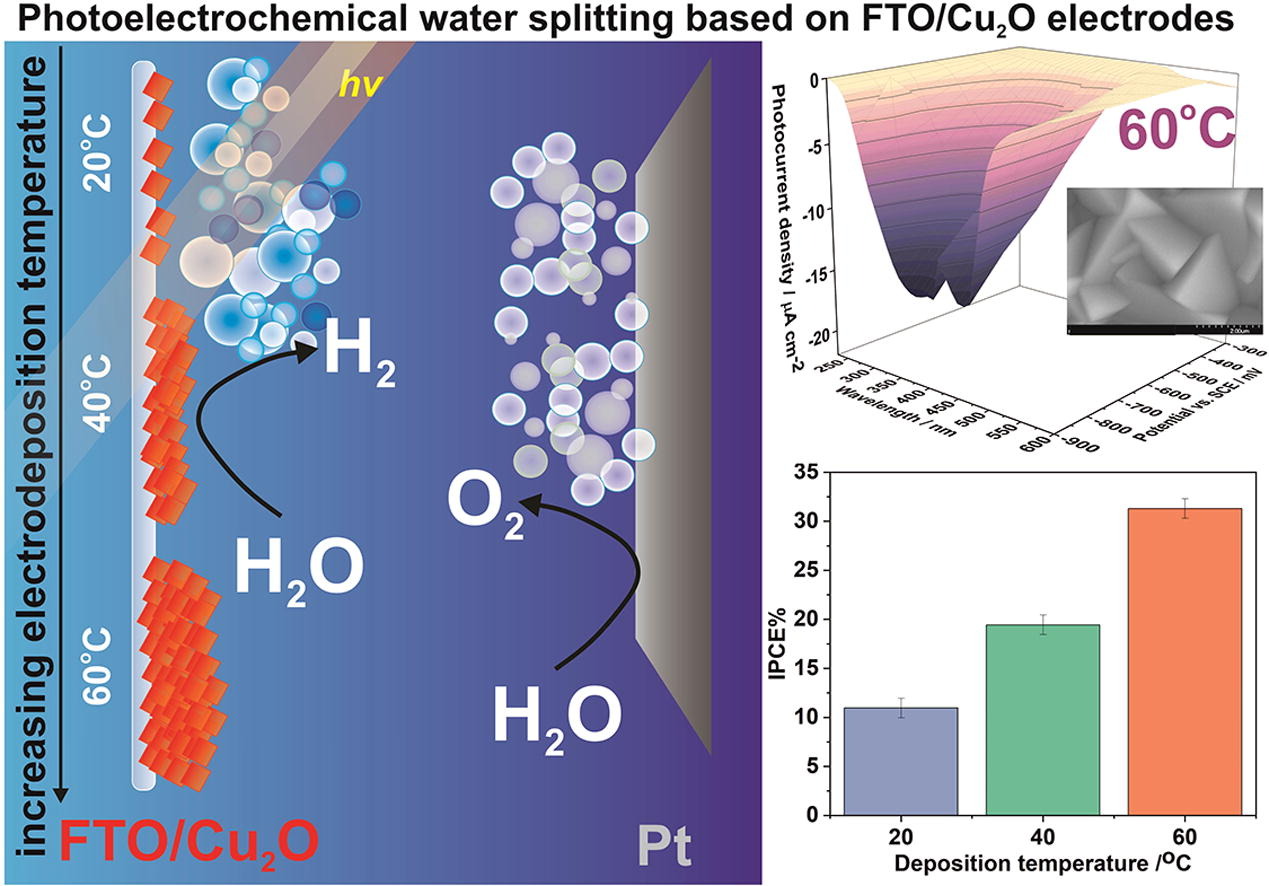
Cuprous oxide (Cu2O) is considered one of the most promising candidates for photocathodes in photoelectrochemical systems due to its favorable properties including p-type conductivity and narrow band gap. Therefore, Cu2O thin films were electrochemically deposited on FTO-coated glass from the bath containing 0.2 M CuSO4 and 3 M lactic acid (pH=12) at the potential of −0.5 V vs SCE for 1 h at three different temperatures (20 °C, 40 °C, and 60 °C). The obtained materials were characterized using various techniques including SEM/EDS, XRD, UV–vis DRS, and EIS measurements. It was found that rising the electrodeposition temperature causes not only an increase in the oxide growth rate but also influences the crystallite orientation preference (the higher preferred orientation of the〈111〉plane was observed at higher temperatures). Photoelectrochemical measurements confirmed the superior performance of the FTO/Cu2O cathode synthesized at the highest studied temperature (60 °C), most likely due to the greater thickness of the deposit as well as higher material texture. On the contrary, the optical and electrochemical band gaps were found to be independent of the temperature applied during deposition. Finally, the possibility of using such kind of electrochemically synthesized FTO/Cu2O photocathodes for solar light-induced ammonia synthesis was also demonstrated.
A. Knapik, K. Syrek, M. Kozieł, L. Zaraska, Cathodic deposition of SnO2 layers on transparent conductive substrates and their photoelectrochemical activity, J. Ind. Eng. Chem. 111 (2022) 380-388.
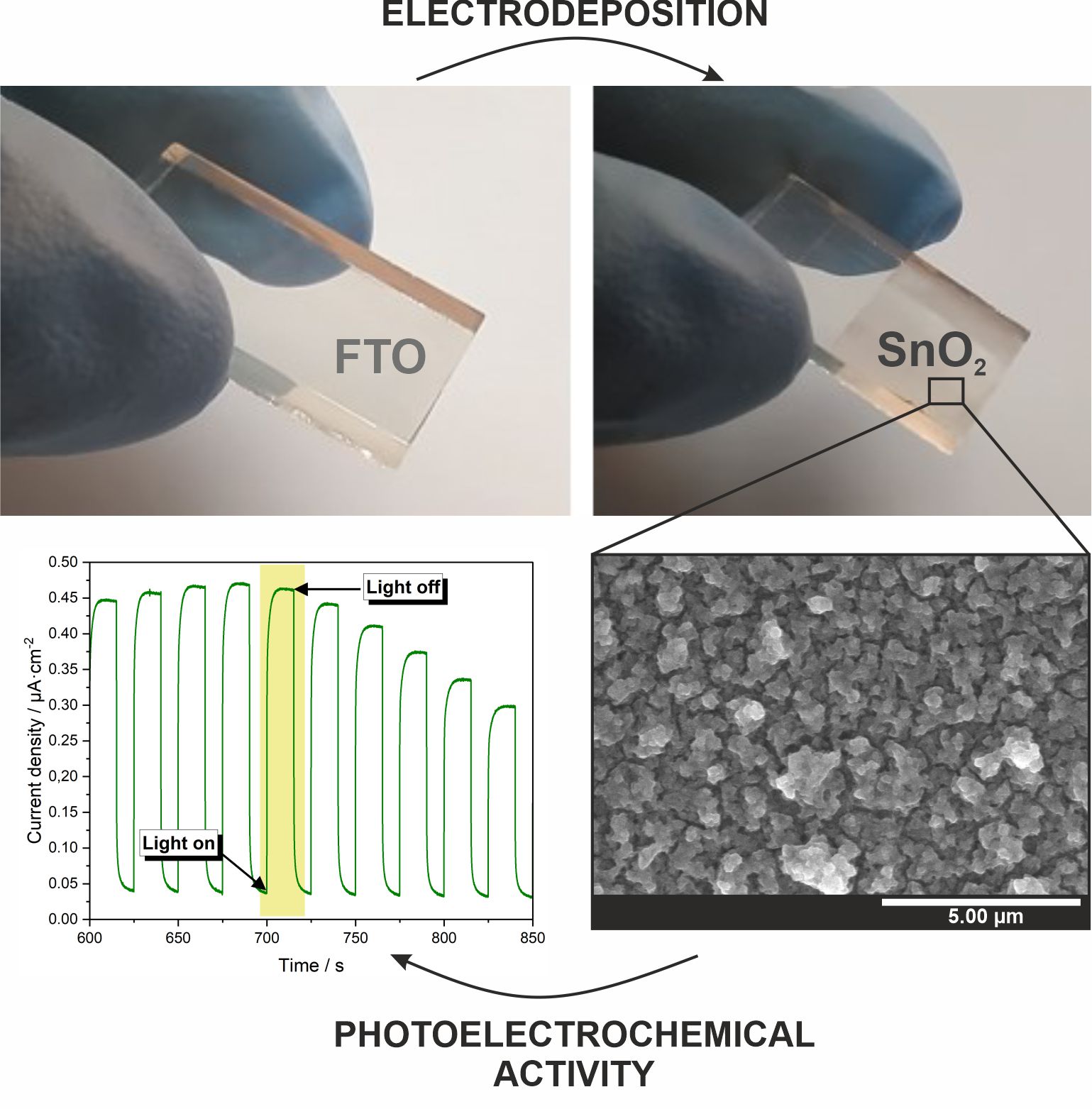
Tin dioxide (SnO2) films deposited on transparent conductive substrates have been considered as promising candidates for photoelectrochemical and photovoltaic applications due to their promising properties such as high electron mobility, stability, and favorable band edges positions. Therefore, a simple electrochemical approach based on the cathodic deposition of SnO2 on a conductive glass substrate was proposed. SnO2 layers were obtained by electrodeposition from SnCl2 solution containing HNO3 at various potentials (from -0.6 V to -1.0 V vs. SCE) for various durations (from 5 min to 180 min). The obtained materials were then annealed in air at 400 °C and characterized. The co-deposition of the metallic phase at the most negative potential was confirmed. However, it was successfully converted to the oxide phase by thermal treatment. The photoelectrochemical activity of the materials during illumination with monochromatic light from the range between 250 and 500 nm was studied. The higher photocurrents were generated by annealed layers, and the most promising photoelectrochemical properties were observed for the sample deposited at -0.8 V vs. SCE for 180 min. It is expected that such a simple electrochemical approach can be an effective strategy for the fabrication of highly efficient photoanodes for photoelectrochemical and photovoltaic applications.